Hi Everyone - There is a message on my home page re the future of this site.
Year 11 CHEMISTRY
The year 11 course covers units one and two of the QCAA Chemistry syllabus. Units three and four are covered in year 12 Chemistry. Year 11 is consider entirely formative and generally covers introductory topics within Chemistry.
![]() Course Overview for Year 11 Chemistry - 2023 version.
Course Overview for Year 11 Chemistry - 2023 version.
Links to the IA's (Internal Assessments)
Data Test Research Investigation Student Investigation
- TERM 1 Sections:
- 1. Atomic Structure & Isotopes
- 5. Measurement and Eerror
- 2. Periodic Table and Trends
- 6. Compounds and Mixtures
- 3. Chemical reactions
- 7. Exo and Endo Rns
- 4. Mole concept
- 8. Introduction to Bonding
TERM ONE
10 weeks (30 lessons, 35hrs max.)
![]() Learning Goals and Success Criteria - for all topics covered in term 1.
Learning Goals and Success Criteria - for all topics covered in term 1.
1. Atomic structure and Isotopes - Week 1 (3 lessons)
![]() Homework sheet 1 - Atomic structure and Isotopes
Homework sheet 1 - Atomic structure and Isotopes
Intro stuff (Success Criteria 1 to 4)
 Atomic Structure Basics - Four slides then a quiz - from Annenberg Learner. Covers atoms and periodicity. Should already know this (We may make some notes...depends). Must do the quiz to check for understanding.
Atomic Structure Basics - Four slides then a quiz - from Annenberg Learner. Covers atoms and periodicity. Should already know this (We may make some notes...depends). Must do the quiz to check for understanding.
 Nuclear Binding Force - Not in our success criteria, but interesting knowledge. This is the last time we will look at the nucleus. Chemistry is all about electrons from here on in.
Nuclear Binding Force - Not in our success criteria, but interesting knowledge. This is the last time we will look at the nucleus. Chemistry is all about electrons from here on in.
 Simulation - Build an Atom - A PhET Simulation. Simple, asks students to build atoms from sub-atomic particles.
Simulation - Build an Atom - A PhET Simulation. Simple, asks students to build atoms from sub-atomic particles.
![]() Relative masses,charges and positions of the subatomic particles - IB video by Richard Thornley
Relative masses,charges and positions of the subatomic particles - IB video by Richard Thornley
![]() Nuclear Symbol Equations - IB video by Richard Thornley
Nuclear Symbol Equations - IB video by Richard Thornley
 Worksheet - Nucear symbol notation - Stolen from California State Uni. Northbridge website here
Worksheet - Nucear symbol notation - Stolen from California State Uni. Northbridge website here
Electron configeration (Success Criteria 5 to 7)
Note there are no success criteria relating to the Bohr or Quantum Mechanical models of atoms. However it is important that students move their thinking from the simple ideas of electron shells (in the Bohr model), to the idea of shells, subshells, and orbitals (in the QM model) because students must learn to apply the Aufbau principle, Hund's rule, and the Pauli exclusion principle. I will use a brief class lecture to move from the bohr model to the QM model without making notes.
 Bohr model to QM model. This is a good (but relatively straight forward) summary, but does not fully explain the shortcomings of the Bohr model and why the QM model is better.
Bohr model to QM model. This is a good (but relatively straight forward) summary, but does not fully explain the shortcomings of the Bohr model and why the QM model is better.
 Suggested (simulation) experiment - Rutherford's Gold foil exp
Suggested (simulation) experiment - Rutherford's Gold foil exp
![]() Worksheet: figuring out the rules for electron arrangement - some Critical Creative Thinking. Use for homework or maybe at the start of a lesson to try to generate some analysis and logical thinking processes.
Worksheet: figuring out the rules for electron arrangement - some Critical Creative Thinking. Use for homework or maybe at the start of a lesson to try to generate some analysis and logical thinking processes.
![]() Electron configurations for atoms and ions up to Z = 36 - an IB video by Richard Thornley. 15 mins long. Step by step process is explained
Electron configurations for atoms and ions up to Z = 36 - an IB video by Richard Thornley. 15 mins long. Step by step process is explained
 Notes and Interactive - Click on "it's elementary" in the top menu. Has slides with notes on Orbitals and writing electron arrangement, plus a "Building elements" interactive after 3 more slides. Good simple interactive - Use to CFU.
Notes and Interactive - Click on "it's elementary" in the top menu. Has slides with notes on Orbitals and writing electron arrangement, plus a "Building elements" interactive after 3 more slides. Good simple interactive - Use to CFU.
 Interactive Periodic Table and electron arrangement - Click on the element and its electron arrangement is illustrated in energy level diagrams
Interactive Periodic Table and electron arrangement - Click on the element and its electron arrangement is illustrated in energy level diagrams
Isotopes (Success Criteria 8 to 10)
![]() Isotopes and Atomic Mass worksheet - a good coverage of knowledge and skill questions for this topic
Isotopes and Atomic Mass worksheet - a good coverage of knowledge and skill questions for this topic
 Notes and a quiz Click Isotopes on the top menu. First page is notes, second is an online five question quiz by Annenberg Learner. Quiz changes each time you reload it.
Notes and a quiz Click Isotopes on the top menu. First page is notes, second is an online five question quiz by Annenberg Learner. Quiz changes each time you reload it.
 Isotope simulation, PhET, Uni. of Colorado. - click "abundance in Nature" and "Symbol" buttons. Set questions for students to list the symbols and abundance of isotopes of certain elements.
Isotope simulation, PhET, Uni. of Colorado. - click "abundance in Nature" and "Symbol" buttons. Set questions for students to list the symbols and abundance of isotopes of certain elements.
![]() Compare the properties of the isotopes of an element - an IB video by Richard Thornley (2 min)
Compare the properties of the isotopes of an element - an IB video by Richard Thornley (2 min)
Finish this section with students constructing a graphic organiser of Atomic structure (CCTS)
2. Periodic Table and Trends - Week 2 (3 lessons)
![]() Homework sheet 2 - Periodic table and Trends
Homework sheet 2 - Periodic table and Trends
 Online pre-test on Periodic table - Click on "Test your skills" in the top menu. By Annenberg Learner. Quite long, 39 questions. Some will be above students, so use this again as a post-test.
Online pre-test on Periodic table - Click on "Test your skills" in the top menu. By Annenberg Learner. Quite long, 39 questions. Some will be above students, so use this again as a post-test.
 Blank graphic organiser (scroll down) - Direct Instruction. Teacher led. Collectively build a list of key terms and graphic organiser from existing knowledge. Two good sites for graphic organisers are here and here
Blank graphic organiser (scroll down) - Direct Instruction. Teacher led. Collectively build a list of key terms and graphic organiser from existing knowledge. Two good sites for graphic organisers are here and here
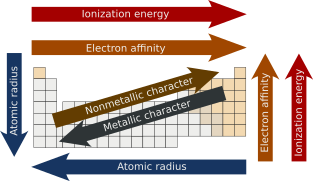
![]() The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity - by Professor Dave. Using the questions on following worksheet as a guide to make notes. Don't have to answer the questions exactly as your notes, but the notes you take should enable you to answer the questions.
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity - by Professor Dave. Using the questions on following worksheet as a guide to make notes. Don't have to answer the questions exactly as your notes, but the notes you take should enable you to answer the questions.
![]() Worksheet to go with video above - use questions on the worksheet to prompt the notes you need from the video.
Worksheet to go with video above - use questions on the worksheet to prompt the notes you need from the video.
 Worksheet on graphing Periodic Trends - a pdf, can do graphing by hand, but challenge students to graph on excel without retyping the data (download, open with word, paste data into excel)
Worksheet on graphing Periodic Trends - a pdf, can do graphing by hand, but challenge students to graph on excel without retyping the data (download, open with word, paste data into excel)
 Periodic table data from Mrbigler.com. - This is an excellent spreadsheet prepared by "Mr Bigler and available from his site here - click some of the bottom tables for trend data.
Periodic table data from Mrbigler.com. - This is an excellent spreadsheet prepared by "Mr Bigler and available from his site here - click some of the bottom tables for trend data.
![]() Trends in Atomic Radii - an IB video by Richard Thornley
Trends in Atomic Radii - an IB video by Richard Thornley
![]() Trends in Electronegativity - an IB video by Richard Thornley
Trends in Electronegativity - an IB video by Richard Thornley
![]() Trends in 1st Ionisation Energy - an IB video by Richard Thornley
Trends in 1st Ionisation Energy - an IB video by Richard Thornley
 Worksheet on Periodic Trends - lots of review and trends practice. Has answers so no stress.
Worksheet on Periodic Trends - lots of review and trends practice. Has answers so no stress.
 Notes for this unit - Direct Instruction. Clearly link succesive Ionisation Energies to electron configeration.
Notes for this unit - Direct Instruction. Clearly link succesive Ionisation Energies to electron configeration.
 Questions on succesive Ion Energies - only a few questions, but excellent for CFU.
Questions on succesive Ion Energies - only a few questions, but excellent for CFU.
 Answers to the worksheet above on succesive Ion Energies
Answers to the worksheet above on succesive Ion Energies
![]() Reactivity Group 1 and 7 - Direct Instruction. Introduce video, discuss with students. Watch again, students summarise. An IB video by Richard Thornley.
Reactivity Group 1 and 7 - Direct Instruction. Introduce video, discuss with students. Watch again, students summarise. An IB video by Richard Thornley.
![]() 5 trends in Group 1 and 7 - discusses specific trends in Group 1 and 7. Students take notes. An IB video by Richard Thornley.
5 trends in Group 1 and 7 - discusses specific trends in Group 1 and 7. Students take notes. An IB video by Richard Thornley.
![]() Changes of ionic to covalent,basic to acidic,of period 3 oxides - Direct Instruction. Introduce video, discuss with students. Watch again, students summarise. An IB video by Richard Thornley.
Changes of ionic to covalent,basic to acidic,of period 3 oxides - Direct Instruction. Introduce video, discuss with students. Watch again, students summarise. An IB video by Richard Thornley.
3. Chemical reactions - Week 3 (2 lessons)
Probably only need one lesson as balancing will be assumed knowledge (no Hwk Sheet)
![]() Observing Chemical change - students can make notes from this for SC 1. Video by Bozeman science (5 min)
Observing Chemical change - students can make notes from this for SC 1. Video by Bozeman science (5 min)
![]() Balancing and states in chemical equations - simple video from Fuse school
Balancing and states in chemical equations - simple video from Fuse school
 Worksheets for balancing - list of printable pdfs, with answers. Two online quizzes are at the bottom
Worksheets for balancing - list of printable pdfs, with answers. Two online quizzes are at the bottom
4. Mole concept & law of conservation of mass - Weeks 3,4,5 (7 lessons)
This topic was covered in year 10 PCS and most students should already have a good background in this topic
![]() Homework sheet 4 - This has a few tough ones. There are a couple which are double starred and these are extension work for those wishing to either drive themselves mad, or exhibit mastery of the mathematical process in this section of the course.
Homework sheet 4 - This has a few tough ones. There are a couple which are double starred and these are extension work for those wishing to either drive themselves mad, or exhibit mastery of the mathematical process in this section of the course.
![]() Calculating Empirical and Molecular formula - 5 mins by FuseSchool. Nice simple intro explaining these two terms and showing calculation of both. In less than 5 mins... sweet!
Calculating Empirical and Molecular formula - 5 mins by FuseSchool. Nice simple intro explaining these two terms and showing calculation of both. In less than 5 mins... sweet!
 Empirical and Molecular formula worksheet. Nice worksheet (only 7 Qs), not mine, stole it from Peoria Public schools in Illinois here, where it has answers.
Empirical and Molecular formula worksheet. Nice worksheet (only 7 Qs), not mine, stole it from Peoria Public schools in Illinois here, where it has answers.
![]() Second Worksheet on calculating Emp and Mol Formula - more questions, less formal phrasing. Not mine, but can't find original author. The first page has Qs, the second page has the answers.
Second Worksheet on calculating Emp and Mol Formula - more questions, less formal phrasing. Not mine, but can't find original author. The first page has Qs, the second page has the answers.
 Simulation - Calculating the empirical formula of Copper oxide
Simulation - Calculating the empirical formula of Copper oxide
 Experiment - determining the empirical formula of MgO - simple, as you would expect. Can use glass wool to stop some of the smoke escaping.
Experiment - determining the empirical formula of MgO - simple, as you would expect. Can use glass wool to stop some of the smoke escaping.
The Mole - Students need to get a really good fundamental understanding of the mole and the basic calculations. This knowledge will be visited frequently in Units 3 and 4 (Yr 12). The videos here are mainly support material and I will not use them in class.
![]() The mole - a series of videos - by Tyler DeWitt. A collection of 11 videos (vids 1,2,5,6 and 8 are most useful)
The mole - a series of videos - by Tyler DeWitt. A collection of 11 videos (vids 1,2,5,6 and 8 are most useful)
![]() The mole - by Brainstorm, essentially the traditional teaching lecture about what a mole is.
The mole - by Brainstorm, essentially the traditional teaching lecture about what a mole is.
![]() Mole and Molar Mass - by JFR Science, good, succinct, and usful intro to the calculation of n.
Mole and Molar Mass - by JFR Science, good, succinct, and usful intro to the calculation of n.
![]() Calculating mass and moles - A tyler Dewitt vid, good simple and clear. Not exactly how I do it in class, but comprehensive.
Calculating mass and moles - A tyler Dewitt vid, good simple and clear. Not exactly how I do it in class, but comprehensive.
![]() Stoichiometry - how to do - My own video (33 mins). First video of mine (a few things to improve) - shows my method for doing these Qs. Covers all the basic types of Stoichiometry questions
Stoichiometry - how to do - My own video (33 mins). First video of mine (a few things to improve) - shows my method for doing these Qs. Covers all the basic types of Stoichiometry questions
![]() mass to mass simple 3 step Stoichiometry questions - need to be able to do these in your sleep!
mass to mass simple 3 step Stoichiometry questions - need to be able to do these in your sleep!
![]() Stoichiometry Worksheet - range of questions. Good intro to exam style questions although none are "extension".
Stoichiometry Worksheet - range of questions. Good intro to exam style questions although none are "extension".
 Experiment - Law of Conservation of Mass - Simple but effective online instructions, reaction between Barium chloride and Sodium sulphate. Tabs at top have supprting video and theory.
Experiment - Law of Conservation of Mass - Simple but effective online instructions, reaction between Barium chloride and Sodium sulphate. Tabs at top have supprting video and theory.
![]() Lead Iodide Exp - excellent Limiting Reagent experiment. I stole this from a site and rewrote quite a bit of it.
Lead Iodide Exp - excellent Limiting Reagent experiment. I stole this from a site and rewrote quite a bit of it.
![]() Limiting Reagent - by Tyler DeWitt.
Limiting Reagent - by Tyler DeWitt.
 Simulation - reactants, products, and leftovers. - PhET by Uni of Colorado.
Simulation - reactants, products, and leftovers. - PhET by Uni of Colorado.
![]() Limiting reagent questions - These are the hardest style of Stoichiometry questions
Limiting reagent questions - These are the hardest style of Stoichiometry questions
![]() How to Solve Problems on Theoretical, Experimental and Percentage Yield - an IB video by Richard Thornley
How to Solve Problems on Theoretical, Experimental and Percentage Yield - an IB video by Richard Thornley
![]() Percentage yield questions - an extra step on the regular stoichiometry qs
Percentage yield questions - an extra step on the regular stoichiometry qs
Measurement uncertainty and Error - Weeks 6,and 7 (6 lessons)
The resources below are pretty much all videos. We will be using our text for practice qurestions and notes. This topic was extensively covered in Year ten, so reviewing those notes would be very useful.
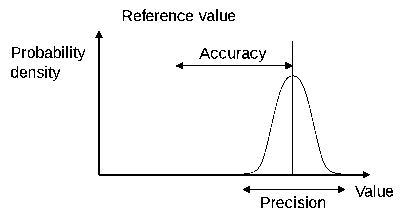
![]() Accuracy and Precision - an IB video by Richard Thornley
Accuracy and Precision - an IB video by Richard Thornley
![]() Worksheet - Accuracy and Precision - not mine. Super Simple - should use as a whole class exercise rather than individual work.
Worksheet - Accuracy and Precision - not mine. Super Simple - should use as a whole class exercise rather than individual work.
![]() State random uncertainty as a uncertainty range - an IB video by Richard Thornley
State random uncertainty as a uncertainty range - an IB video by Richard Thornley
![]() Determine the uncertainties in results - an IB video by Richard Thornley
Determine the uncertainties in results - an IB video by Richard Thornley
![]() State uncertainties as absolute and percentage uncertainties - an IB video by Richard Thornley
State uncertainties as absolute and percentage uncertainties - an IB video by Richard Thornley
![]() The appropriate number of significant figures - an IB video by Richard Thornley
The appropriate number of significant figures - an IB video by Richard Thornley
![]() Describe and give examples of random uncertainties and systematic errors - an IB video by Richard Thornley
Describe and give examples of random uncertainties and systematic errors - an IB video by Richard Thornley
![]() how the effects of random errors (uncertainties) may be reduced - an IB video by Richard Thornley
how the effects of random errors (uncertainties) may be reduced - an IB video by Richard Thornley
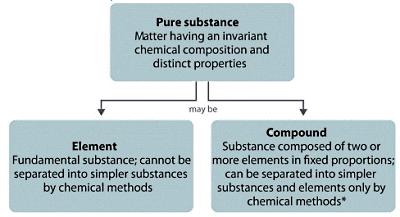
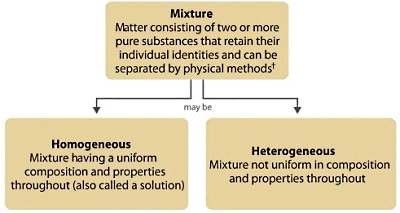
6. Compounds and Mixtures - Week 8 (3 lessons)
May not need the whole 3 lessons as these ideas are not complicated nor need practicing.
7. Exothermic and Endothermic reactions - Week 9 (3 lessons)
This section will be split into 3 distinct lessons. Furstly an introduction to enthalpy, lots of definitions, diagrams, understanding. Secondly will be calculating Change in Enthaply using Bond enthalpies and calorimetry. Third will be a mandatory calorimetry experiment.
Resources for the first lesson - may do the notes and go straight to calculating Change in Enthalpy using bond enthalpy
![]() *Notes for the first lesson - Need only the first 3 pages. We will do some highlighting and discussing, and work through the questions. One of the SC is not covered by these Qs.
*Notes for the first lesson - Need only the first 3 pages. We will do some highlighting and discussing, and work through the questions. One of the SC is not covered by these Qs.
![]() Enthalpy - by crashcourse chemistry. Good Background and overview. Watch but don't take notes
Enthalpy - by crashcourse chemistry. Good Background and overview. Watch but don't take notes
![]() *Worksheet on Enthalpy and Stoichiometry - nice simple questions, good intro. Adapted from http://mspeavychemistryclairemont.weebly.com/uploads/6/0/1/5/60154085/16-3enthalpystoich.pdf
*Worksheet on Enthalpy and Stoichiometry - nice simple questions, good intro. Adapted from http://mspeavychemistryclairemont.weebly.com/uploads/6/0/1/5/60154085/16-3enthalpystoich.pdf
Resources for the second lesson
![]() Calculating Enthalpy change using Bond Enthalpies - fousses on "how" to do the calculation, not the "why" but quite clear and methodical. I recommend seperating the reactant calculation to the left of the page and the products to the right for more structure to your answer.
Calculating Enthalpy change using Bond Enthalpies - fousses on "how" to do the calculation, not the "why" but quite clear and methodical. I recommend seperating the reactant calculation to the left of the page and the products to the right for more structure to your answer.
![]() Calculating Change in Enthalpy for Bond Enthalpies - a one pager edited and stolen from Mr Kent's Chem page here. Practice only simple ones of these.
Calculating Change in Enthalpy for Bond Enthalpies - a one pager edited and stolen from Mr Kent's Chem page here. Practice only simple ones of these.
 *Worksheet on Bond energies - good place to start. Has only one combustion Q though, which are the focus for this unit.
*Worksheet on Bond energies - good place to start. Has only one combustion Q though, which are the focus for this unit.
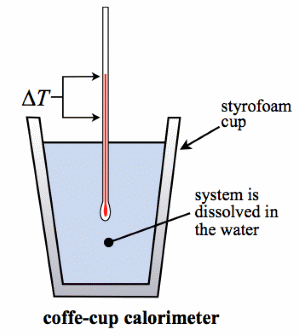
![]() Me teaching Calorimetry - Experimental calculations of change in Enthalpy for reactions
Me teaching Calorimetry - Experimental calculations of change in Enthalpy for reactions
 Calorimetry Worksheet - looks very messy as it has the answers with it.
Calorimetry Worksheet - looks very messy as it has the answers with it.
I recommend doing some of the Fuel calorimetry questions from the two sheets in Section 15 below
Resources for the third lesson
 Classic Zinc in Copper Sulphate - seem well set out, I like the graph extrapolation, nice touch.
Classic Zinc in Copper Sulphate - seem well set out, I like the graph extrapolation, nice touch.
 Calorimetry experiment simulation - probably best to start here, as we can do a "real" fuel experiment in next section (FUELS). Use to gather data, and do calculations.
Calorimetry experiment simulation - probably best to start here, as we can do a "real" fuel experiment in next section (FUELS). Use to gather data, and do calculations.
REVISION - Huge worksheet - range of questions, do not do them all, ask your teacher for some on topic one's
![]() Worksheet on range of Questions - more questions here than you need. We will do only some in class.
Worksheet on range of Questions - more questions here than you need. We will do only some in class.
Data Test Week 9
Data test practice - there are a few sheets here. The first four were done in the week or two prior to week nine. The last practice sheet "revision for data test" provides the most comprehensive set of practice and was the last sheet given out in class (on wednesday)
![]() Data and Questions on the Magnesium Oxide Empirical formula
Data and Questions on the Magnesium Oxide Empirical formula
![]() Data and Questions on periodic trends
Data and Questions on periodic trends
![]() Data and Questions on Successive Ionisation energies
Data and Questions on Successive Ionisation energies
![]() Data and Questions on Periodic trends No.2
Data and Questions on Periodic trends No.2
 *ANSWERS Revision for data test -and yes the errors i made (no-one mention that out aloud!) have been corrected in this copy (and highlighted so you know where they are).
*ANSWERS Revision for data test -and yes the errors i made (no-one mention that out aloud!) have been corrected in this copy (and highlighted so you know where they are).
8. Introduction to Bonding - Week 10 (3 lessons)
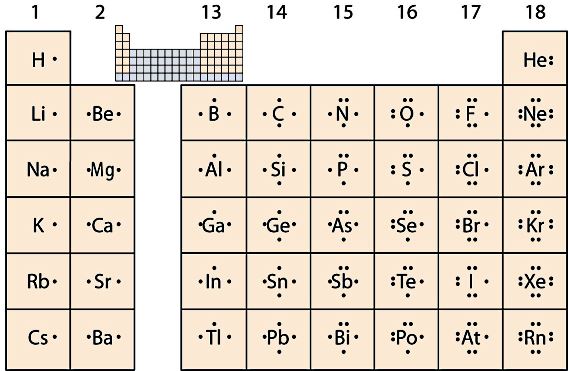
LG 1:Students can write and describe the exchange of electrons in compounds formed from ions (ionic compounds)
Collaborative groups (CCTS), teacher led Direct Instruction - The key question in this LG is "Why do elements react the way that they do?". But to answer this students must first understand... what does "react" mean in chamistry? Works in small groups on this latter question. When the class has a satisfactory answer, try the first "key" question, again in groups. Teacher to make notes on electron exchange and electron sharing (via electron configeration and electronegativity, ion formation, electrostatic attraction) from class discussion. I think it is a good idae to introduce electron exchange (ionic) versus electron sharing (metallic and covalent) The videos below are good for students who want to prepare early, or struggle with the ideas.
![]() Introduction to ionic bonding - creating ions and electron transfer. Part of a very old video series and not one I own, so this is breaking copyright. However I can no longer source this video commercially and despite low resolution it is excellent.
Introduction to ionic bonding - creating ions and electron transfer. Part of a very old video series and not one I own, so this is breaking copyright. However I can no longer source this video commercially and despite low resolution it is excellent.
![]() How ionic lattices form. Part of a very old video series and not one I own, so this is breaking copyright. However I can no longer source this video commercially and despite low resolution it is excellent.
How ionic lattices form. Part of a very old video series and not one I own, so this is breaking copyright. However I can no longer source this video commercially and despite low resolution it is excellent.
![]() Valence electrons and the periodic table - Tyler DeWitt (Simple,Excellent)
Valence electrons and the periodic table - Tyler DeWitt (Simple,Excellent)
![]() Why atoms lose or gain electrons - actually called "octet rule and valence charges" - it claims to be clear and simple and it actually is
Why atoms lose or gain electrons - actually called "octet rule and valence charges" - it claims to be clear and simple and it actually is
![]() Writing chemical (ionic) formulas Tyler Dwitt - good simple introduction
Writing chemical (ionic) formulas Tyler Dwitt - good simple introduction
Checking For Understanding - The next resource is a good checking for understanding worksheet. It is adapted from an exam question released years ago in a C2C exam.
![]() The periodic table on the planet MuAran If you can do this you know all you need to know about electron configeration, electron exchange, ions and ionic formula.
The periodic table on the planet MuAran If you can do this you know all you need to know about electron configeration, electron exchange, ions and ionic formula.
![]() Worksheet Comparing Ionic and Covalent - a one pager, nice simple check for understanding
Worksheet Comparing Ionic and Covalent - a one pager, nice simple check for understanding
LG 2: Students can draw Lewis diagrams of simple ionic and covalent formula
Direct Instruction - Direct instruction on Lewis diagrams is important here, covering both ionic and covalent. Most Lewis diagram worksheets will cover only covalent molecules. Model drawing valence shells, electron counting, electron exchange (ionic), and electron sharing (covalent)
 Excellent -Lewis diagrams - an ACS Chemistry page. Written from a teachers point of view. Has great Video, recommend you Direct Instruction first, show video after or as you teach examples. Has a student activity sheet (right menu, quite long for about 9 Qs), and answers
Excellent -Lewis diagrams - an ACS Chemistry page. Written from a teachers point of view. Has great Video, recommend you Direct Instruction first, show video after or as you teach examples. Has a student activity sheet (right menu, quite long for about 9 Qs), and answers
![]() Lewis diagrams for covalent molecules - crashcourse, great inro and mentions the rules for drawing lewis diagrams, simple molecules only. Hint play at 0.8 speed.
Lewis diagrams for covalent molecules - crashcourse, great inro and mentions the rules for drawing lewis diagrams, simple molecules only. Hint play at 0.8 speed.
![]() Lewis diagrams for covalent molecules - Sciencepost, very useful tutorial, gos thru some simple and then some more complex lewis structures. A good follow up to the crashcourse video.
Lewis diagrams for covalent molecules - Sciencepost, very useful tutorial, gos thru some simple and then some more complex lewis structures. A good follow up to the crashcourse video.
LG 3: Students can explain that basic differences in the physical properties of Ionic and Covalent substances using basic description of lattice and molecular structure
NOTE: LG 3 leads direct to LG1 in the next section - in LG 3 "basic" relates most likely to the fact that chemical bonds hold ionic lattices together, but physical forces hols covalent molecules together. Use this to explain high MPt versus low MPt
![]() Brief notes on Ionic Bonding Good to have in front of you for this lesson or use as study notes
Brief notes on Ionic Bonding Good to have in front of you for this lesson or use as study notes
![]() Covalent Molecular Bonding brief notes - need only page 1 notes (ignore the table of molecule structures)for this LG
Covalent Molecular Bonding brief notes - need only page 1 notes (ignore the table of molecule structures)for this LG
![]() Properties of ionic bonding - not professionally produced but well explained
Properties of ionic bonding - not professionally produced but well explained
![]() Quiz (easy) on Covalent Molecular Bonding
Quiz (easy) on Covalent Molecular Bonding
TERM 2
![]() Learning Goals and Success Criteria - for all topics covered in term 2.
Learning Goals and Success Criteria - for all topics covered in term 2.
9. Bonding and properties - Week 11 (3 lessons)
LG 1: Students can describe and identify the type of bonding present in simple ionic, metallic, and covalent substances.
I use Direct Instruction a lot here as I find this topic is mainly memory work for students - so study notes and remembering are key. They do need to understand the bonding models in order to use them to explain, but any exam question on this will require students to remember the key points of a bonding model in order to explain a property. CCTS will involve interpreting data in order to identify the bonding type, but from there on its memory work.
![]() Notes on all bonding models and properties Good for study notes.
Notes on all bonding models and properties Good for study notes.
![]() Brief notes on Ionic Bonding Good to have in front of you for this lesson or use as study notes
Brief notes on Ionic Bonding Good to have in front of you for this lesson or use as study notes
![]() GCSE Science Chemistry Properties of ionic compounds - not exciting but very on point. Exactly what you need to know and understand and no more.
GCSE Science Chemistry Properties of ionic compounds - not exciting but very on point. Exactly what you need to know and understand and no more.
![]() Metallic bonding Mexus Education - an oldie but a goodie.
Metallic bonding Mexus Education - an oldie but a goodie.
![]() Brief notes on Metallic Bonding
Brief notes on Metallic Bonding
 Properties of ionic and Covalent Online quiz - Doc brown site - good simple revision for properties and bonding - will do as whole class exercise. Note, give the Qs a few secs to load, and questions may not load in all browsers (chrome works,IE not)
Properties of ionic and Covalent Online quiz - Doc brown site - good simple revision for properties and bonding - will do as whole class exercise. Note, give the Qs a few secs to load, and questions may not load in all browsers (chrome works,IE not)
![]() Practice short answer questions on Metals
Practice short answer questions on Metals
![]() Covalent Molecular Bonding brief notes - need only page 1 notes (ignore the table of molecule structures)for this LG
Covalent Molecular Bonding brief notes - need only page 1 notes (ignore the table of molecule structures)for this LG
![]() Simple QUIZ Chemical Bonding Good to do once an introduction of the three bonding models is done
Simple QUIZ Chemical Bonding Good to do once an introduction of the three bonding models is done
![]() Chemical Bonding Questions - great range of multiple choice and short answer questions. Good homework sheet
Chemical Bonding Questions - great range of multiple choice and short answer questions. Good homework sheet
10. FUELS - Week 12 (3 lessons)
These SC and LG are not Knowledge based and this topic is very much research based. I believe the QCAA have this topic here only so it can be included a topic in the research investigation. There are a couple of worksheets with calorimetry questions below, but the bulk of these lesson will be developing research questions about fuels and finding data to answer them. We start the research Task next week.
 *Calorimetry of Fuels worksheet - only 3 questions, but with answers. original is not mine.
*Calorimetry of Fuels worksheet - only 3 questions, but with answers. original is not mine.
 Fuels and Calorimetry - variations in fuel calorimetry questions. Bit harder than simple application of process. Also with answers. Original is not mine.
Fuels and Calorimetry - variations in fuel calorimetry questions. Bit harder than simple application of process. Also with answers. Original is not mine.
STUDENT RESEARCH INVESTIGATION - : weeks 13 - 16 (2 lessons per week, 8 lessons)
Below is the task sheet. By far the hardest part of this research investigation will be finding data to develop a research question. Once you have done this and it is okayed by your teacher, the two supporting documents should make it easier to write your report.
![]() Research investigation TASK SHEET - This is the 2023 version.
Research investigation TASK SHEET - This is the 2023 version.
The next two documents are to support you writing the report for your investigation.
![]() How to write your research Investigation report - this is a key document. It contains recommended heading and sections (subheadings) for your report. Each subheading is explained in terms of what you need to write aboute and the criteria which will be used to evaluate your writing. This doc and the next one work together.
How to write your research Investigation report - this is a key document. It contains recommended heading and sections (subheadings) for your report. Each subheading is explained in terms of what you need to write aboute and the criteria which will be used to evaluate your writing. This doc and the next one work together.
![]() Instrument Specific Marking Guide (ISMG) - What I will be using to mark your Research Investigation - a mark out of 20. Can be confusing. The next document attempts to unpack it for you.
Instrument Specific Marking Guide (ISMG) - What I will be using to mark your Research Investigation - a mark out of 20. Can be confusing. The next document attempts to unpack it for you.
![]() The ISMG EXPLAINED - useful document as it uses some common and simple language to explain each of the criteria in terms of what your teachers are looking for.
The ISMG EXPLAINED - useful document as it uses some common and simple language to explain each of the criteria in terms of what your teachers are looking for.
11. Intermolecular forces - Week 13 and 14 (6 lessons)
LG 1*: Students can explain the relative physical properties of covalent molecular compounds using theories of intermolecular forces.
This is a complex topic, but not simple CCTS, more like a process type problem solving CCTS. I teach this as a combination of process (steps to follow) and logic application with lots of examples. Constant feedback to students is essential, along with Practicing and Deepening. I use lots of impromtu CFU followed by Practicing and Deepening The idea of a Flipped classroom is very useful for this topic.
VSEPR Theory.
![]() Covalent Molecular Bonding brief notes - need only page 2 of the notes for this LG
Covalent Molecular Bonding brief notes - need only page 2 of the notes for this LG
![]() Worksheet VSEPR theory - a "how to" guide. 3 pages, notes, guided practice. My version of how to do this. Check the notes on the front page.
Worksheet VSEPR theory - a "how to" guide. 3 pages, notes, guided practice. My version of how to do this. Check the notes on the front page.
![]() VSEPR Theory - Science post, very methodical and clear but more an "explanation of" rather than a "how to" video.
VSEPR Theory - Science post, very methodical and clear but more an "explanation of" rather than a "how to" video.
![]() VSEPR Theory - Tyler DeWitt, more of a "how to" video, not the same but similar method to mine.
VSEPR Theory - Tyler DeWitt, more of a "how to" video, not the same but similar method to mine.
![]() Practice in VSEPR Theory - Tyler DeWitt
Practice in VSEPR Theory - Tyler DeWitt
Madatory Practical - practice VSEPR theory using 3D model kits
Polarity
I use this video as a basis to make notes. Quite thorough. This is a complex topic and builds upon VSEPR Theory.
![]() Polarity in covalent molecules - Sciencepost, very methodical and clear. 15 mins long.
Polarity in covalent molecules - Sciencepost, very methodical and clear. 15 mins long.
Intermolecular forces
My prefferred technique here is Direct Instruction. I use the flowchart to proceduralise (am I making up words here?) this topic. The video is good preview material or for additional support for students. One document has 6 pages of mainly MC questions, where you will find some Qs to use for CFU and Practice and Deepening.
![]() Intermolecular forces notes - good basics. Work for your study notes is highlighted.
Intermolecular forces notes - good basics. Work for your study notes is highlighted.
![]() Intermolecular forces flowchart - This is an excellent tool for predicting the melting and boiling points of compounds. It may also help you organise you thoughts on the 3 types of Intermolecular bonding
Intermolecular forces flowchart - This is an excellent tool for predicting the melting and boiling points of compounds. It may also help you organise you thoughts on the 3 types of Intermolecular bonding
![]() Intermolecular forces - by Dr Paul McCord. Good coverage and very clear
Intermolecular forces - by Dr Paul McCord. Good coverage and very clear
![]() Questions (tradional exam style)on covalent molecular bonding 6 pages of questions
Questions (tradional exam style)on covalent molecular bonding 6 pages of questions
12/13. Reactions of Acids/Bases and pH - Week 17 and 18 (6 lessons)
This section actually combines two topics in the LG and SC document. These two topics are both knowledge based, although pH requires simple calculations
![]() Notes for Reactions of Acids - an edit from a site called Naturez-Vous. Will go through these notes for knowledge and understanding work.
Notes for Reactions of Acids - an edit from a site called Naturez-Vous. Will go through these notes for knowledge and understanding work.
![]() pH basics - notes and calculations on the basics of pH
pH basics - notes and calculations on the basics of pH
Aqueous Solutions and Molarity - Weeks 19 and 20 (4 lessons)
Success Criteria 71
![]() Water Structure and Hydrogen Bonding - by gmcd1985. Published on Feb 11, 2011. 2 mins.
Water Structure and Hydrogen Bonding - by gmcd1985. Published on Feb 11, 2011. 2 mins.
![]() Properties of Water | Hydrogen Bonding in Water | Biology | Biochemistry - Socratica. Published on Jun 26, 2017. 13 mins.
Properties of Water | Hydrogen Bonding in Water | Biology | Biochemistry - Socratica. Published on Jun 26, 2017. 13 mins.
Success Criteria 72 and 73
 Good source of notes for these SC - from "MrsGetson" site. If it does not load, I stole a copy here , call me paranoid!
Good source of notes for these SC - from "MrsGetson" site. If it does not load, I stole a copy here , call me paranoid!
![]() Distinguish between solute, solvent, solution and concentration - an IB video by Richard Thornley
Distinguish between solute, solvent, solution and concentration - an IB video by Richard Thornley
Success Criteria 74
Mainly direct instruction here as these calculation are very process orientated. These calculations are throughout the course, so reinforce to students that they need to be bullet proof with their confidence. Can use the videos as an intro and to support students who need additional time. Note the SC does not mention dilutions but I think it is appropriate to cover it here (first video).
![]() Molarity, Solutions, Concentrations and Dilutions - Mr. Causey. Published on Jun 18, 2012. 10 mins. Nice and direct.
Molarity, Solutions, Concentrations and Dilutions - Mr. Causey. Published on Jun 18, 2012. 10 mins. Nice and direct.
![]() What is a Part per Million (ppm)? - an IB video by Richard Thornley. 6 mins.
What is a Part per Million (ppm)? - an IB video by Richard Thornley. 6 mins.
![]() Solve Problems Using Concentration, Amount of Solute and Volume - an IB video by Richard Thornley. 2 mins.
Solve Problems Using Concentration, Amount of Solute and Volume - an IB video by Richard Thornley. 2 mins.
![]() Simple Concentration questions Adapted from a "MrsGetson" who had answers. I have edited it significantly down to one page, no answers (we will generate them in class), and converted to word.
Simple Concentration questions Adapted from a "MrsGetson" who had answers. I have edited it significantly down to one page, no answers (we will generate them in class), and converted to word.
![]() Molarity and Stoichiometry - these questions are harder and combine stoichiometry with Molarity, has answers. Not sure of the original author, but is heavily edited.
Molarity and Stoichiometry - these questions are harder and combine stoichiometry with Molarity, has answers. Not sure of the original author, but is heavily edited.
 Suggested Exp - Effect of Conc on Rate - an RSC experiment. Use as a basis for students to develop modifications and practice data analysis techniques.
Suggested Exp - Effect of Conc on Rate - an RSC experiment. Use as a basis for students to develop modifications and practice data analysis techniques.
TERM 3
![]() Learning Goals and Success Criteria - for all topics covered in term 3.
Learning Goals and Success Criteria - for all topics covered in term 3.
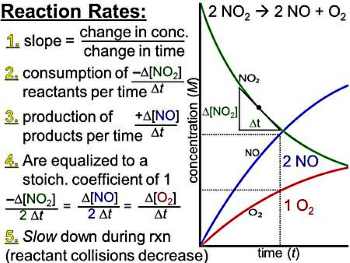
15. Rates of Reaction - Week 23 -27 (7 lessons of 15)
This is mainly a theory section with few calculations. The powerpoint below (first 14 slides only) and the Chemguide site link are all you will need for this unit, except for the experiments.
![]() Reaction rates PwrPt (need only 14 slides, not all) - Will give you a handout of slides 3 to 14. This is 13 year old powerpoint so be nice! This resource covers the theory component of the course and I will use it in class. We will NOT cover the reaction mechanisms part, so you need only the first 14 slides.
Reaction rates PwrPt (need only 14 slides, not all) - Will give you a handout of slides 3 to 14. This is 13 year old powerpoint so be nice! This resource covers the theory component of the course and I will use it in class. We will NOT cover the reaction mechanisms part, so you need only the first 14 slides.
 Reaction Rate Theory - Chemguide site and excellent! It is broken up into different topics so is bitesize and easily navigated.
Reaction Rate Theory - Chemguide site and excellent! It is broken up into different topics so is bitesize and easily navigated.
 Exp - Effect of temp on rate - We will do a version of this one in class (and vary concentration as well). This is a Royal Society of Chemistry site, experiment sheet (scroll down) for measuring the effect of temp on a reaction
Exp - Effect of temp on rate - We will do a version of this one in class (and vary concentration as well). This is a Royal Society of Chemistry site, experiment sheet (scroll down) for measuring the effect of temp on a reaction
 Exp - measuring rate of reaction - Royal Society of Chemistry site, experiment sheet (scroll down) for measuring the rate of a magnesium and acid reaction.
Exp - measuring rate of reaction - Royal Society of Chemistry site, experiment sheet (scroll down) for measuring the rate of a magnesium and acid reaction.
STUDENT EXPERIMENTAL INVESTIGATION : Weeks 23 to 25 ( 8 lsns)
![]() Student Investigation task sheet
Student Investigation task sheet
![]() Write a GREAT student experiment - Latest (2021) version. Step by step, detailed and super, super useful if you take the time (9 pages) to use this how to guide.
Write a GREAT student experiment - Latest (2021) version. Step by step, detailed and super, super useful if you take the time (9 pages) to use this how to guide.
![]() Student Investigation Exemplar - SIMPLE - this is both an exemplar and a learning task. Student have to grade the exemplar (it has a couple of errors) using the criteria of the ISMG on the back page. The individual criteria have been split up and distributed at the relevant part of the report so it should be easier for students. More importantly, after the students try to mark the report it should be very clear to them how obvious they have to be meeting the criteria when they write their own report
Student Investigation Exemplar - SIMPLE - this is both an exemplar and a learning task. Student have to grade the exemplar (it has a couple of errors) using the criteria of the ISMG on the back page. The individual criteria have been split up and distributed at the relevant part of the report so it should be easier for students. More importantly, after the students try to mark the report it should be very clear to them how obvious they have to be meeting the criteria when they write their own report
![]() Student Investigation Exemplar - SENIOR LEVEL - This is also annotated to show how it meets the criteria. However the topic is more complex as are the arguements and logic in the report.
Student Investigation Exemplar - SENIOR LEVEL - This is also annotated to show how it meets the criteria. However the topic is more complex as are the arguements and logic in the report.
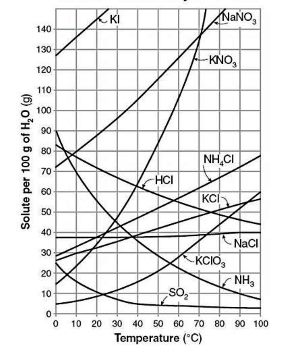
16. Solubility and Identifying ions in solution - Week 30 (3 lessons)
![]() Solubility worksheet - some really good simple and some not simple practice. Edited this sheet from somewhere, can't remember where.
Solubility worksheet - some really good simple and some not simple practice. Edited this sheet from somewhere, can't remember where.
17. Gases - Weeks 29 (3 lessons)
![]() Success Criteria and Learning Goals
Success Criteria and Learning Goals
![]() Homework sheet 5 - Cover this section on gases. Note the number is way out of order (long story!). No answers unfortunately.
Homework sheet 5 - Cover this section on gases. Note the number is way out of order (long story!). No answers unfortunately.
 GASES: notes - from chemguide.co.uk. Quite direct and well explained, not extensive, all you need to know for this course
GASES: notes - from chemguide.co.uk. Quite direct and well explained, not extensive, all you need to know for this course
 Principles of General Chemistry - Chapter 10 Gases. Free to download, a creative commons licence
Principles of General Chemistry - Chapter 10 Gases. Free to download, a creative commons licence
![]() Kinetic Theory Notes and Questions Good summary and 3 excellent questions
Kinetic Theory Notes and Questions Good summary and 3 excellent questions
![]() KINETIC THEORY - Tyler DeWitt discuss Kinetic Molecular Theory (Part 1), good video
KINETIC THEORY - Tyler DeWitt discuss Kinetic Molecular Theory (Part 1), good video
![]() KINETIC THEORY - Tyler DeWitt uses KMT to explain properties of Gases (Part 2), excellent video
KINETIC THEORY - Tyler DeWitt uses KMT to explain properties of Gases (Part 2), excellent video
![]() GAS PRESSURE UNIT CONVERSIONS - Tyler DeWitt shows how to convrt between the units used to measure Gas pressure
GAS PRESSURE UNIT CONVERSIONS - Tyler DeWitt shows how to convrt between the units used to measure Gas pressure
![]() KELVIN - ABSOLUTE ZERO - Tyler DeWitt discusses the idea of absolute zero temperature and Kelvin units
KELVIN - ABSOLUTE ZERO - Tyler DeWitt discusses the idea of absolute zero temperature and Kelvin units
![]() Homework Sheet 1 - written for 2018 course, but in WORD so you can edit as you wish. Has unit conversions, Kinetic Theory and boyle's law.
Homework Sheet 1 - written for 2018 course, but in WORD so you can edit as you wish. Has unit conversions, Kinetic Theory and boyle's law.
Some Direct Instruction and CFU(Teacher modeling and Guided practice) will be needed here as solving the ideal gas law questions are a process orientated activity - make sure to include calculating Molar mass. Opportunity do exist however for students to engage in CCTS by developing ideas about the mathematical relationships. The first resource below is an example.
![]() See Think Wonder- on the graphs which describe Boyle's Law, good exercise. Do as individual, then collaborate, then with your teacher. Do the students see a mathematical relationship?
See Think Wonder- on the graphs which describe Boyle's Law, good exercise. Do as individual, then collaborate, then with your teacher. Do the students see a mathematical relationship?
![]() Homework Sheet Three - covers Ideal gas law and simpler versions of the formula along with units and Kinetic theory. Written for 2018 course, can edit to suit.
Homework Sheet Three - covers Ideal gas law and simpler versions of the formula along with units and Kinetic theory. Written for 2018 course, can edit to suit.
 Answers to Homework Sheet 3 - don't know why but the last two Q's appear unanswered.
Answers to Homework Sheet 3 - don't know why but the last two Q's appear unanswered.
![]() Worksheet - Ideal gas Law questions - not mine. Good simple direct questions - excellent for CFU. Has answers. The site is called teachnlearnchem. it shows as a "not secure" site but I have not had any problems downloading this sheet. The site has other resources as well.
Worksheet - Ideal gas Law questions - not mine. Good simple direct questions - excellent for CFU. Has answers. The site is called teachnlearnchem. it shows as a "not secure" site but I have not had any problems downloading this sheet. The site has other resources as well.
![]() Solving a idel gas law question - Air bags - an IB video by Richard Thornley
Solving a idel gas law question - Air bags - an IB video by Richard Thornley
![]() An ideal gas Law question Good complex practice
An ideal gas Law question Good complex practice
 Simulation of an ideal Gas - recommended by the QCAA, but I couldn't figure it out. Let me know if you know how.
Simulation of an ideal Gas - recommended by the QCAA, but I couldn't figure it out. Let me know if you know how.
 Experiment - detemine the Molar volume of Hydrogen gas - an RSC publication. Can download a teacher and student handout here.
Experiment - detemine the Molar volume of Hydrogen gas - an RSC publication. Can download a teacher and student handout here.
 Gas Law Stoichiometry Worksheet - not mine, was not certain of where this is from, found as a pdf here
Gas Law Stoichiometry Worksheet - not mine, was not certain of where this is from, found as a pdf here
 Experiment on ideal gas law and Air bags - Appears now to be block to Australians ??? no idea.
Experiment on ideal gas law and Air bags - Appears now to be block to Australians ??? no idea.
Check the bottom of this page for additional resources on Gaseswhich are not needed for this course. They have additional worksheets and homework sheets (can harvest questions from them) and work on partial pressure and "real" gases
18. Chromatograpy techniques - Week 15 (3 lessons)
The success criteria in the TLAP does not indicate theoretical depth here, so I am going to teach the theory of chromatography using the partition explaination. This is the explanation used in the YouTube clip below (without the big words!). Key terms - stationary phase, mobile phase, Retardation factor, polarity, adsoption, desorption, eluent, retention time.
![]() How TLC Chromatography works - a vtchemist video. Short and sweet on partition chromatography, uses no terminology.
How TLC Chromatography works - a vtchemist video. Short and sweet on partition chromatography, uses no terminology.
![]() Different types of chomatography - Khan academy. Start basic, discussing paper chromatography, then discusses the other types of chromatography.
Different types of chomatography - Khan academy. Start basic, discussing paper chromatography, then discusses the other types of chromatography.
![]() calculating Rf values - by the khan academy. Nice. I've been made obselete!
calculating Rf values - by the khan academy. Nice. I've been made obselete!
 Chromatography in brief - very brief notes, quite hard for studnets to interpret, but good teaching notes. Has a good brief summary of each type of chromatography.
Chromatography in brief - very brief notes, quite hard for studnets to interpret, but good teaching notes. Has a good brief summary of each type of chromatography.
 Chromatography quiz - Click the "review questions" in the left hand menu. 15 questions, quite hard.
Chromatography quiz - Click the "review questions" in the left hand menu. 15 questions, quite hard.
The rest of this page is currently under construction. The resources on this page from this point forward are suited to an older QCAA course. The following resources will not be updated or organised and eventually deleted as the new QCAA curriculum becomes bedded down.
![]() Worksheet on classifying matter - very simple classification of substances according to some of the vocabulary students need to develop in this section.
Worksheet on classifying matter - very simple classification of substances according to some of the vocabulary students need to develop in this section.
 Text on Acids and Bases - Chemlibre text. Comprehensive for notes
Text on Acids and Bases - Chemlibre text. Comprehensive for notes
GOAL: Describe a chemical reaction in terms of moles of reactant or product
The following three vids all address the mole. find one which makes sense to you. The mole is not a concept we actually assess you on, but is a vital concept for you to understand for the rest of the unit.
![]() The mole - by Brainstorm
The mole - by Brainstorm
![]() The mole and Molar mass - by JFR Science - very good
The mole and Molar mass - by JFR Science - very good
![]() The mole - a series of videos - by Tyler DeWitt. A collection of 11 videos (vids 1,2,5,6 and 8 are most useful)
The mole - a series of videos - by Tyler DeWitt. A collection of 11 videos (vids 1,2,5,6 and 8 are most useful)
![]() Simple mass, concentration, gas volume (STP) calculations - not involving a reaction (no balanced equation needed)
Simple mass, concentration, gas volume (STP) calculations - not involving a reaction (no balanced equation needed)
![]() Calculation flowchart for basic stoichiometric Q's - my version of a maths map for beginning Chemists. Very customisable so change as you like. Updated copies with more complicated calculations appear in later units.
Calculation flowchart for basic stoichiometric Q's - my version of a maths map for beginning Chemists. Very customisable so change as you like. Updated copies with more complicated calculations appear in later units.
![]() Stoichiometry (9mins) - by JFR Science - excellent and simple. At 3:45 the basic 3 step outline of stoichiometry is shown - very very important.
Stoichiometry (9mins) - by JFR Science - excellent and simple. At 3:45 the basic 3 step outline of stoichiometry is shown - very very important.
![]() Also Stoichiometry (15 mins) - Mad Scientist - slower, more detailed and also excellent and simple
Also Stoichiometry (15 mins) - Mad Scientist - slower, more detailed and also excellent and simple
![]() Stoichiometry - how to do - My own video (33 mins). First video of mine (a few things to improve) - shows my method for doing these Qs. Covers all the basic types of Stoichiometry questions
Stoichiometry - how to do - My own video (33 mins). First video of mine (a few things to improve) - shows my method for doing these Qs. Covers all the basic types of Stoichiometry questions
![]() Complex question sheet - range of complex but closed questions on stoichiometry
Complex question sheet - range of complex but closed questions on stoichiometry
![]() Percentage Composition questions - these are a stand alone type of question, a different style to the others
Percentage Composition questions - these are a stand alone type of question, a different style to the others
TERM 3: Burn Baby Burn
This unit introduces the nomenclature and properties of organic chemistry – primarily alkanes, alkenes, alkynes and alcohols. Fermentation and distillation reactions will be performed in the laboratory. The concepts of enthalpy and intropy are introduced and students will qualitatively and quantitatively assess the suitability of ethanol and petrol as fuel sources.Assessment: Response to Stimulus (exam conditions)
![]() IUPAC Nomenclature - for Alkanes, Alkenes, Alkynes, Alcohols, and Halogenated compounds.
IUPAC Nomenclature - for Alkanes, Alkenes, Alkynes, Alcohols, and Halogenated compounds.
Intro to Enthalpy and Stoichiometry
Gases
Additional resources:![]() Homework Sheet two - covers Charles's Law and the Combined Gas Law
Homework Sheet two - covers Charles's Law and the Combined Gas Law
![]() Homework Sheet Four - revision for the work so far
Homework Sheet Four - revision for the work so far
![]() Homework Sheet Five - Dalton's Law and Graham's Law
Homework Sheet Five - Dalton's Law and Graham's Law
![]() Homework Sheet Six - Real Gases
Homework Sheet Six - Real Gases
 Questions on Boyle's Law and Charles's Law - a page of questions on each law. Good practice.
Questions on Boyle's Law and Charles's Law - a page of questions on each law. Good practice.
 Notes on Boyle's Law - good source of notes
Notes on Boyle's Law - good source of notes
 Iowa State Uni - java showing collection of data and graphing of Boyle's law
Iowa State Uni - java showing collection of data and graphing of Boyle's law
![]() Boyle's Law - Tyler DeWitt
Boyle's Law - Tyler DeWitt
 Notes on Charles's Law - good source of notes
Notes on Charles's Law - good source of notes
 Iowa State Uni - java showing collection of data and graphing of Charles's law
Iowa State Uni - java showing collection of data and graphing of Charles's law
![]() Charles's Law - Tyler DeWitt
Charles's Law - Tyler DeWitt
![]() Combined Gas Law Law - Tyler DeWitt
Combined Gas Law Law - Tyler DeWitt
 Notes and Questions on the combined gas law - worksheet, pdf, pretty straightforward
Notes and Questions on the combined gas law - worksheet, pdf, pretty straightforward
![]() Which gas equation do I use? - Tyler DeWitt, reduce the confusion!
Which gas equation do I use? - Tyler DeWitt, reduce the confusion!
![]() IDEAL GAS Equation - Crash Course Chemistry, good discussion of all gas laws
IDEAL GAS Equation - Crash Course Chemistry, good discussion of all gas laws
![]() IDEAL GAS Law Practice Questions - Tyler DeWitt
IDEAL GAS Law Practice Questions - Tyler DeWitt
 Worksheet on Ideal Gas Law - has worked answers
Worksheet on Ideal Gas Law - has worked answers
![]() Worksheet on the Gas Laws so far Good basic coverage of the Laws up to the Ideal Gas equation. No real complex questions. Should be done as early revision
Worksheet on the Gas Laws so far Good basic coverage of the Laws up to the Ideal Gas equation. No real complex questions. Should be done as early revision
![]() PARTIAL PRESSURE - Crash Course Chemistry, good example - boy, he can talk!
PARTIAL PRESSURE - Crash Course Chemistry, good example - boy, he can talk!
 Worksheet on Partial Pressures - has worked answers
Worksheet on Partial Pressures - has worked answers
![]() Root Mean Squared Velocity of a gas - thatchemguy. Technically not part of our course but an elegant example of using units to understand formulas
Root Mean Squared Velocity of a gas - thatchemguy. Technically not part of our course but an elegant example of using units to understand formulas
![]() Applying Graham's law - Brightstorm. Nice simple explanation of using Graham's law to calculate Molar Mass of a gas
Applying Graham's law - Brightstorm. Nice simple explanation of using Graham's law to calculate Molar Mass of a gas
![]() Graham's law - Crash Course. A lot of background in 11 minutes, gives a good overview and tackles graham's law and diffusion at the end.
Graham's law - Crash Course. A lot of background in 11 minutes, gives a good overview and tackles graham's law and diffusion at the end.
 CHEM GUIDE - Real vs Ideal gases - lots of detail but a very good coverage of real gases
CHEM GUIDE - Real vs Ideal gases - lots of detail but a very good coverage of real gases
![]() Real versus ideal gases - IsaacsTEACH. Explains differences between ideal and real gases
Real versus ideal gases - IsaacsTEACH. Explains differences between ideal and real gases
REVISION
 Revision Summary and Questions - downloadable pdf. Good for the basic maths component of the course. Most questions are C and B level
Revision Summary and Questions - downloadable pdf. Good for the basic maths component of the course. Most questions are C and B level
 Revision Questions scan - pdf, good range of mathematical qs, especially q's 60 and 61 which are very good A level practice. Bit awkward looking as it is a cut and paste and scanned in.
Revision Questions scan - pdf, good range of mathematical qs, especially q's 60 and 61 which are very good A level practice. Bit awkward looking as it is a cut and paste and scanned in.
 Worked solutions to Q60 and Q61 - pdf, several ways to do these questions - these solutions show one way.
Worked solutions to Q60 and Q61 - pdf, several ways to do these questions - these solutions show one way.
 High level Revision Questions - key questions are 3, 6, 7, 9, 10, 11, 12, 13, 14, 15. and yes these are all A level questions, some of which have been on past exams.
High level Revision Questions - key questions are 3, 6, 7, 9, 10, 11, 12, 13, 14, 15. and yes these are all A level questions, some of which have been on past exams.
![]() Periodic Table and Mendeleev Crash Course
Periodic Table and Mendeleev Crash Course
![]() Elements and reactivity - 3 page worksheet covering key ideas in this section
Elements and reactivity - 3 page worksheet covering key ideas in this section
 Flowchart for naming Chemical compounds This is a web based link from North Thurston Public School. If you search for the powerpoint it will download directly.
Flowchart for naming Chemical compounds This is a web based link from North Thurston Public School. If you search for the powerpoint it will download directly.
 Balancing Equation - "how to" and Q sheets A comprehensive text and video guide to balancing chem eqns. Scroll down to the bottom of this page and there are several links to worksheets with practice questions and answers
Balancing Equation - "how to" and Q sheets A comprehensive text and video guide to balancing chem eqns. Scroll down to the bottom of this page and there are several links to worksheets with practice questions and answers
 QUIZ on Balancing Equations 10 question multiple choice quiz.
QUIZ on Balancing Equations 10 question multiple choice quiz.
![]() Unknown White Powder Experiment - experimental procedure, practice for the assessment piece.
Unknown White Powder Experiment - experimental procedure, practice for the assessment piece.
![]() Electronic configeration (review) - worksheet: complete the electronic comfigeration of the first 20 elements
Electronic configeration (review) - worksheet: complete the electronic comfigeration of the first 20 elements
![]() Bohr model of the atom - an older video, but a very comprehensive explaination of a complex idea
Bohr model of the atom - an older video, but a very comprehensive explaination of a complex idea
![]() How Atoms bond: Ionic bonding An NBC/NSF video but presented at the Science 360 Video site (loads faster at this site) - Really good simple introduction
How Atoms bond: Ionic bonding An NBC/NSF video but presented at the Science 360 Video site (loads faster at this site) - Really good simple introduction
![]() Quantum Mechanical view of the Atom - Excellent
Quantum Mechanical view of the Atom - Excellent

CHEMISTRY: Downloads and links
 Periodic Table - see the top tabs
Periodic Table - see the top tabs
 Periodic Table - see History & Trends tabs
Periodic Table - see History & Trends tabs
![]() Balancing Chemical Equations Video 1
Balancing Chemical Equations Video 1
![]() Balancing Chemical Equations Video 2
Balancing Chemical Equations Video 2
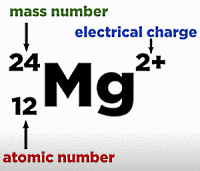
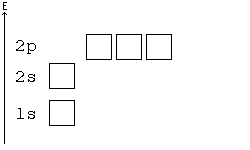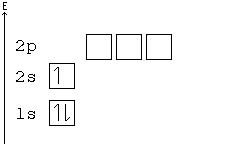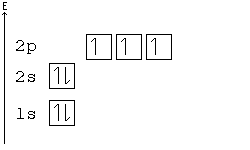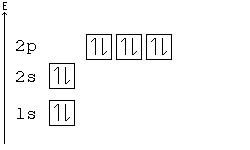
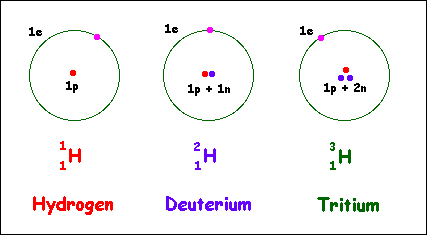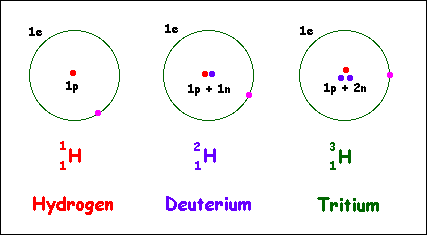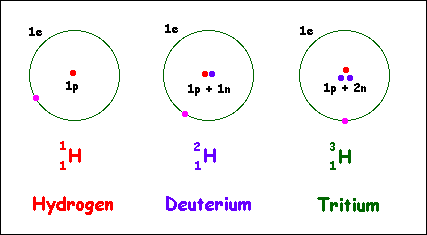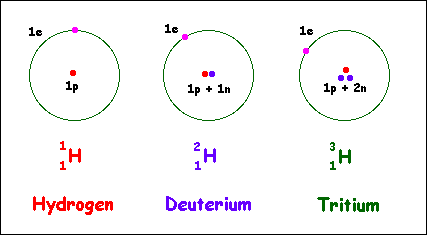
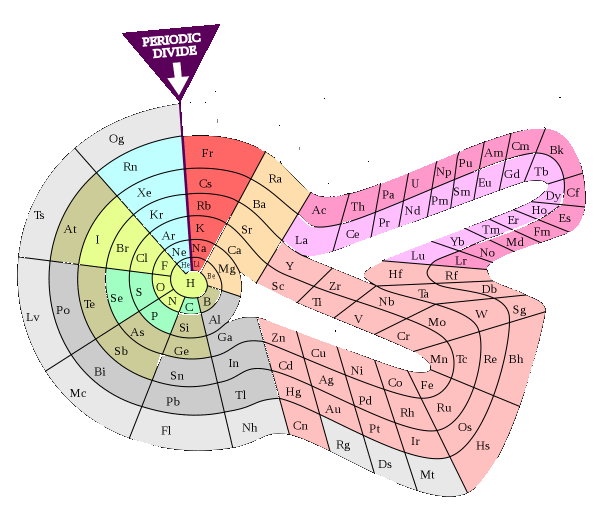 Alternative Periodic table (source = Wikipedia).
Alternative Periodic table (source = Wikipedia).
The following three videos would be good if you miss class or need to reinforce the notes we cover in class
![]() Enthalpy and Change in Enthalpy - by Brightstorm - copy for notes on Enthalpy
Enthalpy and Change in Enthalpy - by Brightstorm - copy for notes on Enthalpy
![]() Thermochemical Equations - by Brightstorm - copy for notes on Thermochemical equations
Thermochemical Equations - by Brightstorm - copy for notes on Thermochemical equations
![]() Energy diagrams - by Brightstorm - Copy. After the video practice explaining the energy diagram to the person behind you. Together figure out a description and write this down as notes beside the diagram
Energy diagrams - by Brightstorm - Copy. After the video practice explaining the energy diagram to the person behind you. Together figure out a description and write this down as notes beside the diagram
![]() Reaction of acids and Carbonates - a free science lesson video
Reaction of acids and Carbonates - a free science lesson video
![]() Reaction of acids and Metals - a free science lesson video
Reaction of acids and Metals - a free science lesson video
![]() Reaction of acids and Bases - Not actually called this, need only the first 3/4s of the video. A free science lesson video
Reaction of acids and Bases - Not actually called this, need only the first 3/4s of the video. A free science lesson video